Recently, the team of Professor Lijie Zhou of the School of the College of Chemistry and Environmental Engineering, Shenzhen University published a latest research paper entitled Investigating cake layer development and functional genes in formate- and acetate-driven heterotrophic denitrifying AnMBRs on the Chemical Engineering Journal (IF:15.1, JCR Chemistry Region 1, TOP Journal). The team's associate professor Lijie Zhou is the first correspondent of the paper, the master student, Nan Dong, is the first author, and Shenzhen University is the first author unit and communication unit.

Canonical denitrification has been used to treat nitrate-laden wastewater produced by the fertilizer, explosives, and metal finishing sectors. Traditional carbon sources amended to drive heterotrophic denitrification are not only expensive, but can inadvertently contribute to operational CO2. It is necessary to find new green carbon sources. formate, a one-carbon molecule, has recently gained attention in both green chemistry and environmental engineering fields. Formate is envisioned as the centerpiece of a carbon-neutral bioeconomy. Electrochemical CO2 gas fixation stands out as a promising method for carbon-neutral formate synthesis. Currently, the use of formate in wastewater treatment has been documented. However, it is not an ideal carbon and energy source for many bacteria. Currently, the utilization of formate in driving heterotrophic denitrification remains underexplored in the literature. Further to the sustainable wastewater treatment viewpoint, Anaerobic Membrane Bioreactors (AnMBRs) offer distinct advantages over conventional activated sludge processes in wastewater treatment. Among the diverse membrane types developed for AnMBRs, flat sheet membrane modules are gaining traction for their small footprint, easy replacement of membranes, and highly effective treatment. However, AnMBRs are not without challenges. Membrane fouling, characterized by pore blocking and cake layer formation, impedes the widespread adoption of AnMBRs.
Responding to the above issues, in this study, we investigated using sodium formate (RMB 2800.00/ton, industrial grade) as an unconventional carbon and energy source for heterotrophic denitrification. Sodium acetate (RMB 5400.00/ton, industrial grade) was used as a conventional but expensive carbon source in WWTP as the control experiment. In particular, we assessed membrane fouling and functional genes in the formate-fed AnMBR in comparison with acetate-fed AnMBR under low and high C/N ratios (i.e., 2.9, 3.7), to explore whether formate is advantageous in the AnMBR nitrogen removal process. In this study, we utilized a standard commercial membrane designed for laboratory applications, with the objective of offering new insights for small-scale wastewater treatment plants in China, particularly in land-constrained and highly-populated urban areas such as Shenzhen, China. In the future, we will conduct large-scale studies in similar wastewater treatment scenarios based on the knowledge gained in this study.
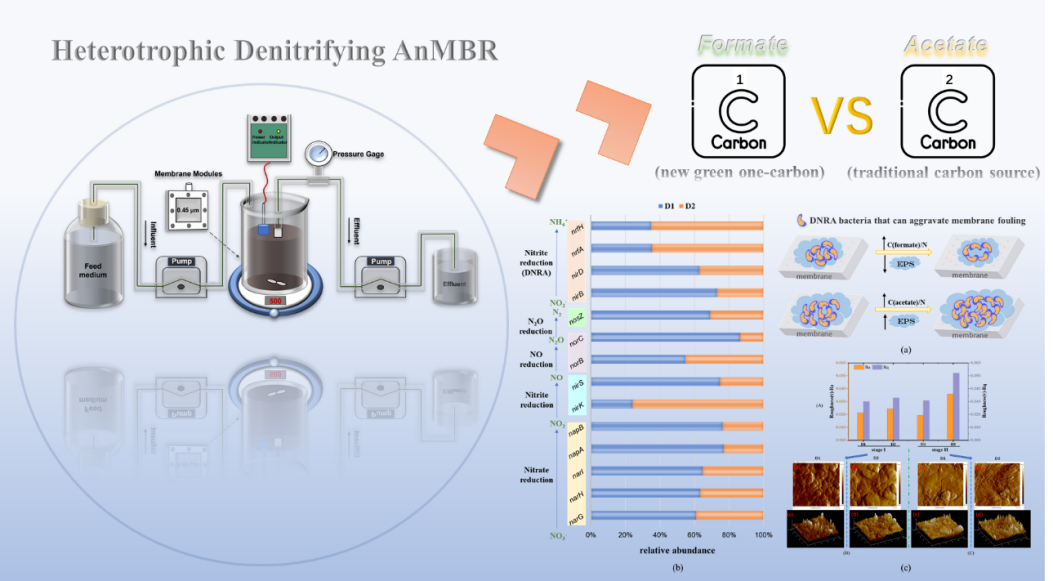
Fig 1 The abstract figure.
Fig. 2a presents the TMP variations in the two AnMBRs. Using formate as the sole carbon and energy source to grow denitrifying bacteria resulted in a much slower TMP increase than a conventional acetate carbon source. Fig. 2b showed the suspension viscosity of acetate-fed sludge (average 80 m Pa.S) is significantly higher than that of formate-fed sludge (average 65 m Pa.S). High viscosity is positively connected to the fouling membrane surface and reduced membrane flux. AFM images showed (Fig. 2c), both Ra and Rq values of the acetate-fed AnMBR were higher than its counterpart, the formate-fed AnMBR. EPS adhesion may be the main trigger and mechanism that causes the cake layer to be compact or loose, its particles can block the membrane pores or form a cake layer during long-term membrane filtration. Fig. 2d showed that the protein content of EPS in the cake layer of both AnMBRs was almost absent. On the other hand, polysaccharides and nucleic acid contents in the acetate-fed cake layers were significantly higher than in the formate-fed cake layer. More interestingly, polysaccharide and nucleic acid contents in the formate-fed cake layers decreased when the C/N ratio increased. The phenomenon was completely opposite in the acetate-fed cake layers and unexpected. The possible cause for this phenomenon may be due to the different metabolic pathways of formate and acetate.
Membrane fouling is the major obstacle to promoting and applying membrane technology in wastewater treatment. Membrane replacement is necessary due to membrane fouling, contributing to approximately 50 % of the operational cost in MBR. The above results showed that formate had less membrane fouling than acetate, which can reduce the frequency of membrane replacement and its operational cost. This study demonstrated the performance of lab-scale membranes, providing insights into the fouling mechanisms relevant to wastewater treatment. In the future, full-scale and pilot-scale membrane studies will be conducted to further expand upon and validate these results.

Fig. 2 TMP (a) and viscosity (b) variations of AnMBR during operation;(c)AFM characterization of membrane scale;(d)The variations of EPS (D1: formate-fed; D2:acetate-fed).
16S rRNA genes and metagenomic sequencing was applied to analyze microbial populations and functional genes in the cake layer taken from the membrane modules. EPS played great roles in biofilm formation, and the exoP, pelABCDEFG, pslAEFGHJ, vpsMNO, wza, gfcE are exopolysaccharide biosynthesis-related genes (https://www.kegg.jp/kegg/pathway.html). Carbohydrate-active enzymes (CAZymes) are extremely important in industrial processes for the removal of biofilms and exopolysaccharides (EPS), including glycoside hydrolases (GHs)、glycosyltransferases (GTs)、polysaccharide lyases (PLs)、carbohydrate esterases (CEs) and auxiliary activities (AAs) (http://www.cazy.org/).
At the phylum level (Fig. 3a), Bacteroidetes decreased from 33% to 12% between Stage I and II, while Proteobacteria increased from 20% to 29% in the formate-fed AnMBR cake layer sample. For the acetate-fed AnMBR cake layer sample, Bacteroidetes increased from 42% to 56%, while Proteobacteria and Firmicutes decreased from 19% to 5% and from 9% to 5%, respectively. Firmicutes and Proteobacteria have been reported to reduce the membrane fouling properties by effectively breaking down macromolecules and aromatic protein substances. MAG_7 (phylum: Firmicutes), MAG_16, and MAG_34 (phylum: Proteobacteria) all contain CAZYmes, which are more predominant in the formate-fed AnMBR. MAG_1 (belongs to phylum Cloacimonadota) became the dominant MAG in formate-fed in stage Ⅱ, accounting for 30% (Fig. 3(b)). Members from this phylum are known to degrade organic matter with four or more carbon atoms via beta-oxidation and are capable of using hydrogen or formate as energy sources. MAG_1 does not contain genes for EPS synthesis but contains CAZymes.
Lentimicrobium (MAG_2) decreased from 10% to 1.8% in the formate-fed AnMBR, but increased from 17% to 38% in the acetate-fed AnMBR. Lentimicrobium is a carbohydrate-fermenting bacterium. Moreover, it was noticed, at the family level, that Anaerolineae (MAG_3) decreased from 7.6% to 3.0% in formate-fed, increased from 10% to 15% in acetate-fed, it might have a significant impact on accelerated sludge floc formation through excretion of extracellular PS or as pioneer species. Lentimicrobium and Anaerolineaceae are both DNRA-capable bacteria and can synthesize EPS, which can lead to more severe membrane fouling in acetate-fed AnMBR. Meanwhile, they also contain CAZymes to degrade complex carbohydrates. Thus, we further calculated the summed abundance of EPS biosynthesis genes and CAZYmes for formate- and acetate-fed, it can be seen that formate-fed AnMBR has fewer EPS biosynthesis genes and more enzymes that degrade complex EPS (Fig. 4(a)). This result aligns with the observation that less fouling developed when formate was used as the carbon source for denitrification.
Nitrogen functional genes shows that formate-fed AnMBR has more DEN genes (Fig. 4(b)). With the ratio of C/N increased, acetate-fed AnMBR possibly accumulated more DNRA-capable bacteria, while the formate-fed AnMBR possibly accumulated more denitrifying-capable bacteria (Fig. 4(c)). Fig. 4(d) shows the principal component analysis (PCA) based on KEGG. The most important is that formate-fed and acetate-fed have different trends. Formate-fed decreases in both PC1 and PC2, acetate-fed increases in PC1 and decreases in PC2. This may also indicate the different membrane fouling trends formed in two reactors.

Fig. 3 Relative abundance of the top 15% of species at Phylum (a) and MAG (b) levels (D1: formate-fed; D2:acetate-fed).

Fig. 4 (a) The relative abundance of genes related to EPS biosynthesis and CAZYmes; (b) The percentage of nitrogen cycle genes involved in denitrification and DNRA; (c) With the C/N ratio increased, the effect of formate and acetate on DNRA bacteria that can aggravate membrane fouling and EPS changes; (d) PCA plot of bacterial community (D1: formate-fed; D2:acetate-fed).
In this study, we found formate, which can be produced from CO2, has excellent potential for heterotrophic denitrification under the laboratory scale. Formate-fed AnMBR exhibited significantly better TN removal with more DEN genes and lowered membrane fouling with more CAZymes (a significant rise in the Candidatus Cloacimonadota and Azospirillum population). However, the acetate-fed AnMBR was dominated by Anaerolineae and Lentimicrobium genera with more DNRA genes and EPS biosynthesis genes, which may lead to the emergence of NH4+-N in the effluent and more severe membrane fouling. Full/pilot scale membrane will be also carried out in similar wastewater treatment in future, on purpose to better bridge the gap between laboratory-scale results in this study and practical applications.
This work is supported by Guangdong Basic Sports Applied Basic Research Fund and Shenzhen Natural Science Fund.
See full text link: https://www.sciencedirect.com/science/article/pii/S1385894724011082
