Dr. Ben Wang from Shenzhen University, Dr. Tiantian Xu from the Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, and Professor Li Zhang from The Chinese University of Hong Kong achieved a significant breakthrough in biomimetic robot design and motion control. The team developed magnetically actuated blood hydrogel fiber robots—derived from patients’ own blood and featuring nematode-inspired structures with multimodal biomimetic locomotion—for precise and minimally invasive drug delivery to deep intracranial regions (Figure 1). This work was published in Nature Biomedical Engineering on May 1, 2025, with Dr. Ben Wang as first and co-corresponding author. The project spanned over three years, with full participation from undergraduate students Zhicheng Ye (Class of 2020, now a PhD student at CUHK) and Jiajun He (now a master’s student at Shenzhen University).
(origin from Nature Biomedical Engineering website)
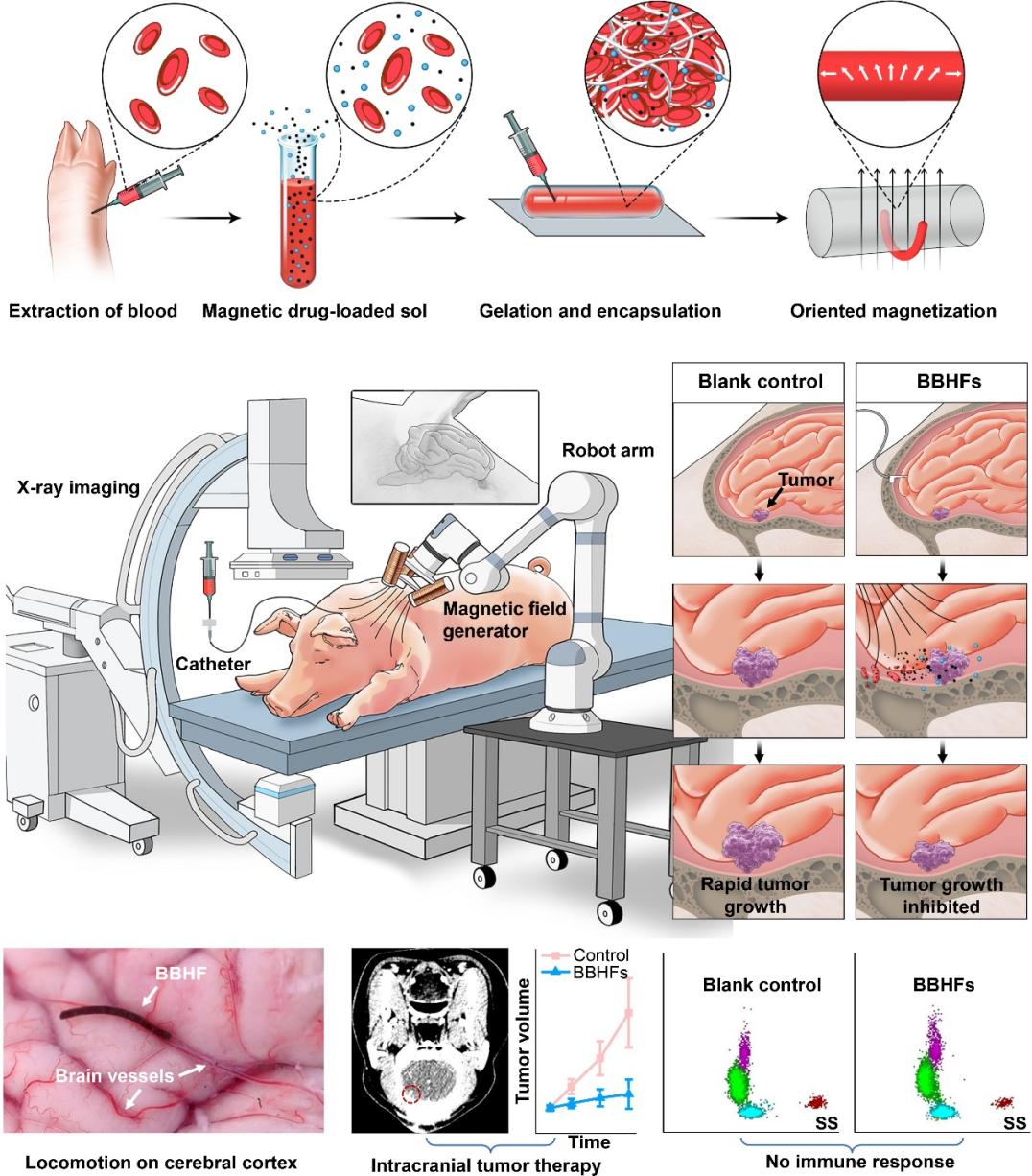
Figure 1. Hydrogel fiber robots derived from the patient’s own blood for deep intracranial control and delivery.
Brain tumors remain among the most formidable challenges in medicine, often located in regions that are difficult to access with current surgical and therapeutic methods. While advances in radiotherapy, chemotherapy, and surgery have improved outcomes, tumors near critical structures still present significant risks. Motivated by the need for safer, more precise, and patient-specific treatments, our recent work introduces magnetically driven biohybrid blood hydrogel fibers (BBHFs)—a novel platform for targeted drug delivery, real-time imaging, and immune evasion.
Inspiration for the Research
This project was inspired by both clinical needs and technological opportunities. Gliomas, for example, frequently develop in areas where surgery risks damaging vital structures like the optic nerves. Existing drug delivery systems struggle with immune activation and the blood-brain barrier. We sought a solution that could overcome these barriers with precise control and high biocompatibility.
Our previous work on nanorobots for thrombosis (Science Advances 2024, 10, adk8970) led us to develop a patient-derived material with a modulus similar to brain tissue and magnetic swimming abilities, ideal for navigating narrow brain spaces. These properties made it a strong candidate for intracranial tumor therapy, as it avoids immune rejection by using autologous material.
Development Challenges
Developing BBHFs involved several hurdles. Material selection was critical; we used the patient’s own blood to maximize biocompatibility and minimize immune response, while carefully incorporating magnetic particles without compromising mechanical or biological properties. Designing precise locomotion in cerebrospinal fluid—much slower than blood flow—required careful tuning of the magnetic actuation system. Integrating real-time X-ray fluoroscopic tracking added complexity, necessitating fine coordination between actuation and imaging for safe navigation through the brain.
Key Breakthroughs
Despite challenges, several breakthroughs marked our progress. Successfully encapsulating and on-demand releasing doxorubicin within BBHFs was pivotal. By applying a strong magnetic field, we achieved precise drug release at tumor sites.
Another milestone was demonstrating BBHF navigation and drug delivery in pig models, validating the system’s potential for personalized intracranial therapy.
Future Directions
BBHFs open new avenues for medical robotics and targeted therapies. The use of patient-derived materials for personalized intervention could be extended to other diseases. Remote control and real-time tracking of soft robots promise further advances in minimally invasive procedures. Future work will focus on enhancing BBHF locomotion, testing additional therapeutics, scaling for clinical trials, and integrating advanced imaging like MRI to complement X-ray guidance.
Conclusion
This study marks a significant step toward personalized, minimally invasive therapies. The success of BBHFs highlights the promise of combining soft robotics, hydrogels, and magnetic control for targeted treatment. We hope our work encourages further innovation in tackling complex medical problems, ultimately improving patient care and realizing new possibilities in medicine.
论文信息:Ben Wang†*, Jie Shen†, Chenyang Huang†, Zhicheng Ye, Jiajun He, Xinyu Wu, Zhiguang Guo, Li Zhang*, Tiantian Xu*, Magnetically-driven biohybrid blood hydrogel fibres for personalized intracranial tumour therapy under fluoroscopic tracking. Nature Biomedical Engineering 2025, DOI: 10.1038/s41551-025-01382-z.
论文链接:https://www.nature.com/articles/s41551-025-01382-z
