Recently, the research team led by Prof. J. Paul Chen at the College of Chemistry and Environmental Engineering, Shenzhen University, published a research paper titled “Phase-Regulated Interfacial Polarization in High-Entropy Materials Enhances Fenton-like Oxidation and Self-Induced Coagulation for Environmental Remediation” in Advanced Functional Materials (impact factor 19; CAS JCR Q1, TOP journal). Master student Tenghui Jin is the sole first author, Assistant Professor Wei Qu and Prof. J. Paul Chen are the corresponding authors. Shenzhen University is the first corresponding affiliation.

With the acceleration of industrialization and urbanization, persistent organic pollutants and emerging contaminants continue to accumulate in water bodies. Their stable chemical structures, high toxicity, and bioaccumulative potential pose severe threats to ecosystems and public health. Conventional biological treatments and standard physico-chemical methods often struggle to achieve efficient degradation, particularly for recalcitrant pollutants containing strong electron-withdrawing groups. In this context, advanced oxidation processes based on reactive oxygen species are considered one of the most promising technologies for deep treatment. However, the widespread application and long-term operational stability of traditional heterogeneous catalysts are constrained by inherent limitations such as insufficient active site exposure, sluggish electron transfer kinetics, susceptibility to deactivation, and metal leaching.
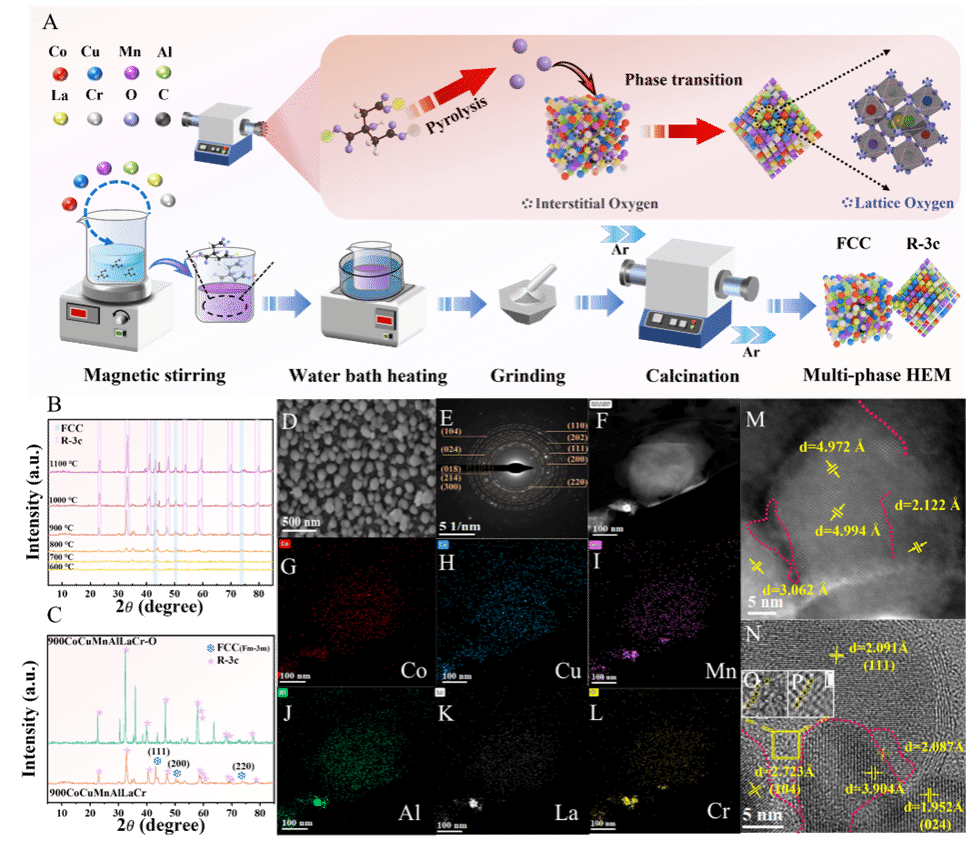
To overcome these challenges, the research team designed and synthesized a multi-component high-entropy material (HEM) comprising cobalt, copper, manganese, aluminum, lanthanum, and chromium (CoCuMnAlLaCr). By precisely controlling the calcination temperature, they successfully induced the formation of a rhombohedral phase (R-3c) within the base face-centered cubic (FCC) structure, creating a dual-phase material with abundant interfaces. A significant built-in electric field forms at these phase interfaces due to differences in work function, which greatly enhances charge separation and transport, thereby dramatically boosting the activation efficiency of peroxymonosulfate (PMS). Experimental results demonstrate that this dual-phase material can achieve a remarkable removal rate of over 93.6% for the herbicide atrazine (ATZ) within just 2 minutes, while maintaining high efficiency across a broad pH range of 3–10. Notably, the incorporation of manganese triggers a novel self-induced coagulation mechanism: Mn2+ ions released during the reaction are oxidized by reactive oxygen species to form MnO2, which aggregates into a three-dimensional network of flocs. These flocs can effectively adsorb and co-precipitate reaction intermediates while also capturing trace leached metal ions from the solution. This synergistic "oxidation-coagulation" process enhances purification and effectively prevents secondary pollution. Further studies confirm that the catalytic system exhibits broad-spectrum removal capability for various types of organic micro-contaminants and maintains strong anti-interference performance against common anions and natural organic matter present in water. The catalyst retained over 90% removal efficiency after 11 consecutive cycles, demonstrating excellent stability and practical utility.
This work not only overcomes the limitations of conventional single-phase HEMs in terms of catalytic activity and reaction pathways but also, through ingenious phase engineering and elemental synergy, constructs a multifunctional water treatment platform integrating rapid oxidation, self-purifying coagulation, and metal ion recycling. This strategy provides important insights for designing the next generation of efficient, green, and sustainable environmental catalysts, potentially advancing the practical application of high-entropy materials in water treatment and broader environmental remediation fields.
This research was supported by multiple funding sources, including the National Natural Science Foundation of China, funds from the Department of Science and Technology of Guangdong Province, the Department of Education of Guangdong Province, the High-Level Talent Start-up Funds of Shenzhen University, and the Scientific Research Foundation for Young Scholars.
Original article: https://doi.org/10.1002/adfm.202520146
