The research team led by Associate Professor Yizhen Liu from the College of Chemistry and Environmental Engineering at Shenzhen University has published a research paper titled "From Lab to Home: Ultrasensitive Rapid Detection of SARS-CoV-2 with a Cascade CRISPR/Cas13a-Cas12a System Based Lateral Flow Assay" in the Nature Index journal Analytical Chemistry (a TOP journal in the first category of the Chinese Academy of Sciences). Full article link https://pubs.acs.org/doi/10.1021/acs.analchem.4c02726 .
Associate Professor Yizhen Liu and Associate Researcher Yong Chen are the corresponding authors of the paper, and graduate student Ronghuan Hu is the first author. Shenzhen University is the sole corresponding institution. This research was strongly supported by the National Key R&D Program of China, the Guangdong Provincial Natural Science Foundation General Program, and the Shenzhen Science and Technology Program.
Background of the Innovation
During the COVID-19 pandemic, home testing emerged as a rapid, convenient, and low-cost diagnostic approach, playing a crucial role in public health management. However, commercially available antigen test kits often suffer from insufficient sensitivity, leading to false-negative results. Although RT-PCR offers high sensitivity, it requires laboratory settings and is thus unsuitable for home use. The team led by Dr. Yizhen Liu developed a novel detection method combining a CRISPR cascade system with lateral flow immunochromatographic analysis. This method enables highly sensitive detection of SARS-CoV-2 without the need for nucleic acid amplification, effectively addressing the limitations of existing diagnostic techniques.
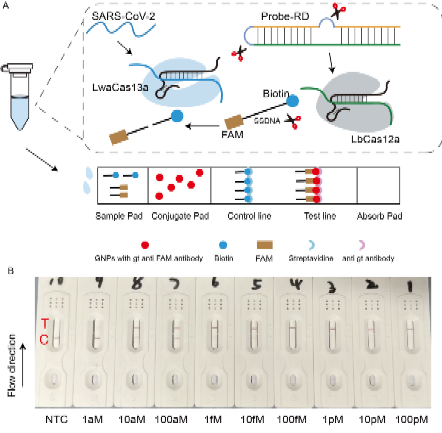
Figure 1. Schematic Diagram of the CLEAR System Principle
Research Highlights
1.Innovative CLEAR Detection System: This study introduces a point-of-care (POC) detection method that is highly sensitive and does not require nucleic acid amplification. Nasopharyngeal swab samples from COVID-19 patients are pre-treated with a viral nucleic acid rapid release agent for just 3-5 minutes, followed by a 30-minute reaction with CLEAR reagents at 33°C. The results are displayed on a paper-based lateral flow strip, enabling quick and straightforward detection.
2.Ultra-high Sensitivity and Specificity: The CLEAR system can detect specific SARS-CoV-2 RNA sequences within 30 minutes, with a limit of detection (LOD) as low as 1 aM. For clinical samples with a CT value below 34.93, CLEAR achieves 100% concordance in both positive and negative results, significantly enhancing the feasibility and reliability of home testing.
3.Accurate Typing Detection: Utilizing programmable crRNA design, this system accurately identifies single nucleotide mutations, such as K417N, K417T, and L452R, enabling non-amplification-based typing of SARS-CoV-2 variants. The CLEAR system demonstrated excellent performance in typing both standard and clinical samples, highlighting its potential for detecting various respiratory pathogens.
4.Convenience and Cost-effectiveness: Designed as a home testing tool, the CLEAR system is easy to use, cost-effective, and reduces contamination risk and detection time compared to traditional PCR methods, making it ideal for use in resource-limited settings.
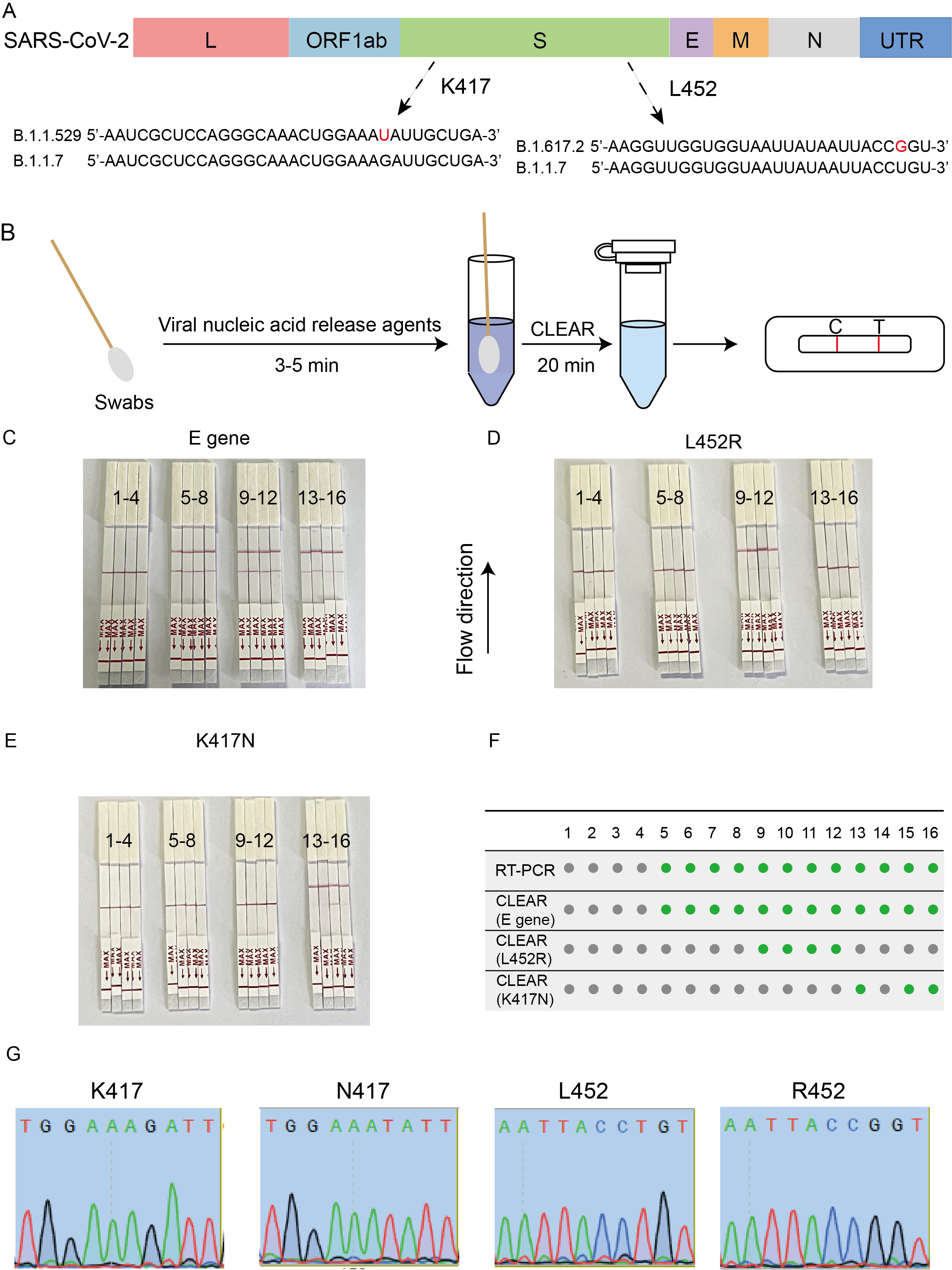
Figure 2. Typing Detection of Clinical Samples Using the CLEAR System
Future Prospects
This study not only provides a novel solution for home molecular testing but also demonstrates the significant potential of CRISPR technology in public health and home diagnostics. In the future, CRISPR-based detection technologies are expected to expand to the detection of other pathogens, supporting broader health monitoring and disease prevention efforts. The development of this technology represents a critical step forward in achieving highly sensitive and reliable disease detection outside of traditional laboratory settings.
