The team of Associate Professor Fan Liangdong from the College of Chemistry and Environmental Engineering, Shenzhen University, published a research paper entitled "In situ reconstruction of proton conductive electrolytes from self-assembled perovskite oxide-based nanocomposite for low temperature ceramic fuel cells" in " Chemical Engineering Journal " (Chinese Academy of Sciences Category 1, TOP Journal). Master's student Ye Weimin and laboratory technician Hu Qicheng are the co-first authors of the paper, Associate Professor Fan Liangdong is the only corresponding author of the paper, and Shenzhen University is the first and sole corresponding institute.

Research background:
Low-temperature solid oxide fuel cells (LT-SOFCs, also known as ceramic fuel cells) are an efficient electrochemical device that directly converts the chemical energy of various fuels into electrical energy, with high energy conversion efficiency and unique fuel flexibility characteristic. It is considered one of the most advantageous clean energy conversion devices in the context of the world's carbon neutrality goals. However, the widespread application of LT-SOFCs suffers from some limitations, especially the lack of electrolyte materials with high conductivity and high manufacturing costs. Researchers have explored different methods to improve ionic conductivity, including creating oxygen vacancies through cation doping and developing semi-ionic conductors. The latter one, initially a partially electronic conductor, transforms into an electronic insulator under fuel cell conditions. In addition, previous studies highlighted the impact of the synthesis methods and related technical parameters on the physical and chemical properties such as phase structure, specific surface area, and surface and interfacial chemistry, and consequently the electrochemical performance of the resulting materials. The self-assembly strategy has attracted increasing attention due to its ability to synthesize nanocomposites with uniform phase distribution, rich heterogeneous interfaces, and strong interfacial bonding through simple processing, and holds potential for the synthesis of highly ion-conducting electrolyte materials.
Article introduction:
A novel semiconductor-based heterostructure material was developed through a lithium doping-induced self-assembly method from apparent perovskite SLxFN material and used as a key component for SOFC electrolyte operating at low temperatures. The newly formed phase of LiFeO2 shows excellent hydration capabilities. The heterostructure is in situ reconstructed into an oxide/oxide-carbonate/hydroxide nanocomposite under fuel cell conditions, presenting significant proton conductivity.
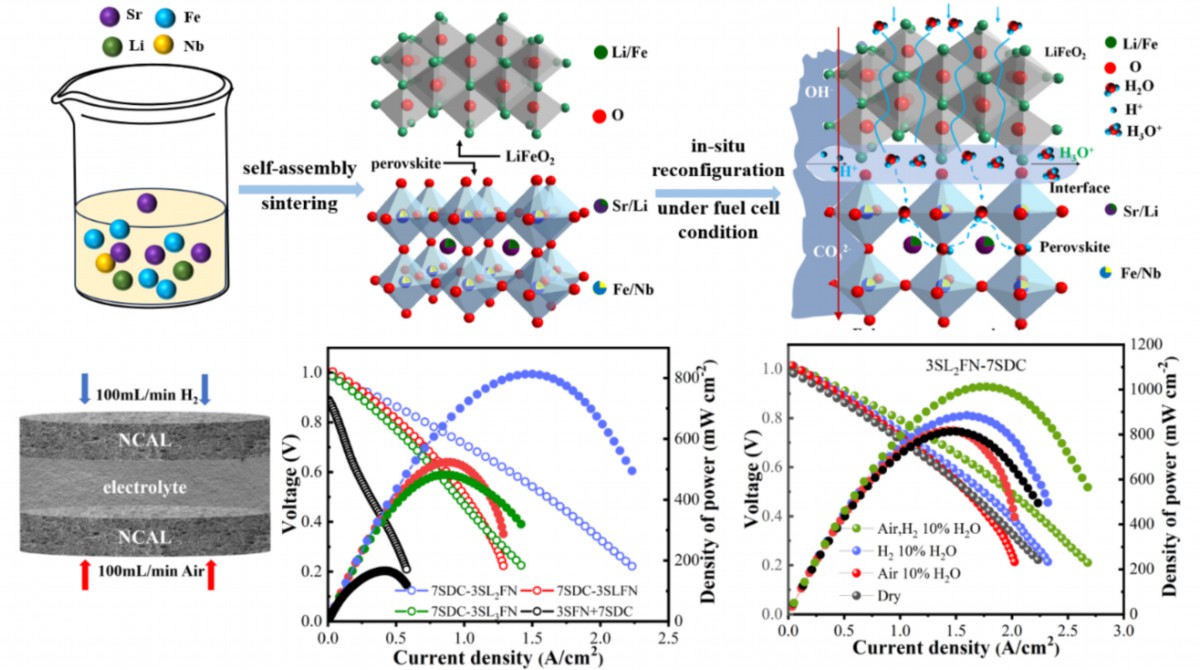
Figure 1. Graphic abstract
Research Highlights:
#1: LiFeO2+SFN semiconductor dual-phase material was obtained from apparent SLxFN perovskite precursor through lithium doping-induced self-assembly method. The increased bulk oxygen vacancy concentration through Li+ substitution and improved hydration capability induced by LiFeO2 contribute to the enhanced ion conductivity in the hybrid.
#2: LiFeO2 and SFN as the key material of SOFC are further in situ reconstructed into a micro-nano heterostructure electrolytic material under the fuel cell atmosphere, forming a LiFeO2-SFN-carbonate hybrid. Rich heterogeneous interfaces, interface defects, and enhanced hydration capacity improve ion conductivity. The ionic conductivity reaches 0.185S/cm, and the peak power density of LT-SOFC single cell reaches 1012mW/cm2 at 550oC.
#3: Studies on the hydration effect, oxygen ion blocking functional layer adding, and concentration cell experiments helped dig out the nature of conducted ion, which is proton transportation through the micro-nano heterostructure electrolyte at low temperatures.
Prospective:
Starting from perovskite semiconductor materials, the study integrates the self-assembly and in-situ phase reconstruction technology to synthesize a new super-ion conductive electrolyte material for low-temperature ceramic fuel cell, which provides new opportunities to achieve efficient electrochemical energy conversion at the reduced temperature and sheds light on new ideas and methodology for the design and synthesis of new electrolyte materials.
This research was financially supported by the National Natural Science Foundation of China, the Basic and Applied Basic Research Program of the Department of Science and Technology, Guangdong Province, and the Taipei University of Technology-Shenzhen University cooperative research project.
Full-text link: https://doi.org/10.1016/j.cej.2024.154977.
