Recently, assistant professor Xiujun Yu’s team from the College of Chemistry and Environmental Engineering at Shenzhen University published the latest research progresses about electrical conductivity of octahedral metallo-supramolecular cages on journal 《Angew. Chem. Int. Ed.》.

A series of metallo-supramolecular octahedral cages were constructed via coordination-driven self-assembly of Ni2+ and Co2+ cations and a truxene-derived tripyridyl ligand L, following our established strategy, to explore host-guest interactions and electrical conductivity at single-molecule level and aggregated bulk state (Figure 1).
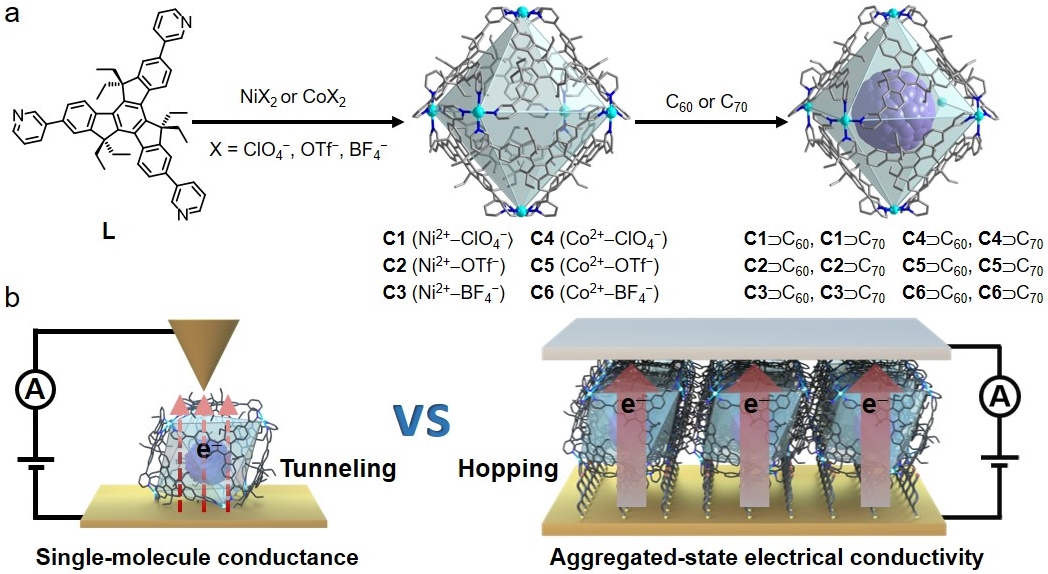
Figure 1. (a) Self-assembly of octahedral metallo-cages and cage-fullerene complexes. (b) Electrical conductivity measurements of metallo-supramolecular ensembles at single-molecule level and aggregated state as well-defined films.
The metallo-cages and the cage-fullerene complexes are comprehensively characterized by electrospray ionization-mass spectrometry (ESI-MS), traveling wave ion mobility-mass spectrometry (TWIM-MS), single-crystal X-ray diffraction (SCXRD), and nuclear magnetic resonance (NMR) spectroscopy. Significantly, the encapsulation of fullerene notably enhances the structural stability of metallo-cages, thereby enabling the systematical study of electrical conductivity at both single-molecule level and aggregated state.
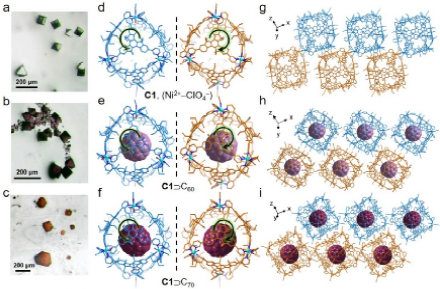
Figure 2. X-ray crystallography study of metallo-cages and cage-fullerene complexes. (a-c) Images of C1, C1ÉC60, and C1ÉC70. (d-f) Crystal structures of C1, C1ÉC60, and C1ÉC70 with a pair of enantiomers. (g-i) Packing modes of C1, C1ÉC60, and C1ÉC70 (hydrogen atoms, solvents and disordered counteranions are omitted for clarity).
Single crystals of cages and cage-fullerene complexes that are suitable for X-ray diffraction were obtained by diffusion of ether/n-hexane mixture (v/v, 1:1) into the corresponding assembly solutions. The resulting crystals displayed distinct colors, viz., black-green for C1-C3, pink for C4-C6, purple for C60 incorporated cages, and reddish-brown for C70 encapsulated cages. Compared to the formation of conglomerates with spontaneous resolution during the crystallization of Cu2+ octahedral cages, all crystallization of Ni2+ and Co2+ cages and cage-fullerene complexes, however, formed racemic compounds (Figures 2). The average cavity diameters of C1, C2, and C3 are 1.42 nm, 1.28 nm, and 1.44 nm, respectively, suggesting the potential for accommodating fullerenes with van der Waals diameters of approximately 1.0 nm.
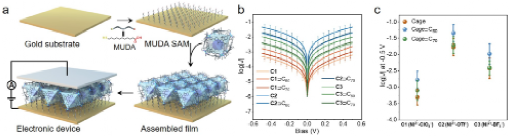
Figure 3. Electrical conductivity study of metallo-cages and cage-fullerene complexes at aggregated state in the form of assembled films. (a) Scheme of electronic device fabrication. (b, c) log|J|-V curves (b) and plots of log|J| at -0.5 V (c) for the assembled films of metallo-supramolecular ensembles with different counterions.
We could systematically evaluate the electrical conductivity of these ensembles at both single-molecule level and aggregated bulk state (as well-defined films). Our findings reveal that counteranions and fullerene guests play a pivotal role in determining the electrical conductivity of aggregated state, while such effects are less significant for single-molecule conductance (Figure 3). Both counteranions and fullerenes effectively tune the electronic structures and packing density of metallo-supramolecular assemblies, and facilitate efficient charge transfer between the cage hosts and fullerenes, resulting in a notable one order of magnitude increase in electrical conductivity of the aggregated state (Figure 4). In the single-molecule conductance, the effects of metal cations, counteranions and fullerenes are less significant owing to the relatively weak tunneling ability.
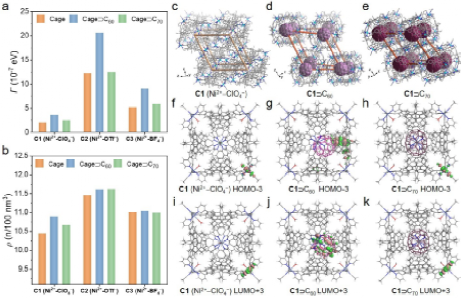
Figure 4. (a, b) Electrode-molecule coupling (Γ, estimated through fitting the experimental J-V data with the single energy level model) and packing density (ρ) of metallo-supramolecular ensembles with different counteranions. (c-e) Simplified packing modes of C1, C1ÉC60, and C1ÉC70 to estimate packing density shown in Figure 6b (hydrogen atoms, solvents and disordered counteranions are omitted for clarity). The modes are extracted by connecting the centroids of eight adjacent octahedral assemblies. (f-h) HOMO-3 of C1, C1ÉC60, and C1ÉC70. (i-k) LUMO+3 of C1, C1ÉC60, and C1ÉC70.
The authors acknowledge the financial support from National Natural Science Foundation of China, the Developmental Fund for Science and Technology of Shenzhen, and the Guangdong Province “Pearl River Talents Plan” Innovative and Entrepreneurial Teams Project.
Article details: Shuai Lu, † Ziang Zhang,† Yiying Zhu, Ye Tao, Quanjie Lin, Qian Zhang, Xin Lv, Lei Hua, Zhi Chen, Heng Wang, Gui-Lin Zhuang, Qian-Chong Zhang,* Cunlan Guo,* Xiaopeng Li, Xiujun Yu*. Enhancing Effect of Fullerene Guest and Counterion on the Structural Stability and Electrical Conductivity of Octahedral Metallo-Supramolecular Cages. Angew. Chem. Int. Ed., 2024, e202410710.
Full-text link: https://doi.org/10.1002/anie.202410710.
